
Introduction: Tyrosine kinase inhibitors (TKIs) have dramatically improved the standard of care for patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP). In the 5-year follow-up of the phase 3 DASISION study (NCT00481247), dasatinib was associated with faster and deeper molecular responses than imatinib as first-line therapy for CML-CP (Cortes J et al. J Clin Oncol 2016). The NCCN guidelines recommend that multiple factors independent of risk score should be considered when selecting TKI therapy, including the presence of comorbidities. More than 50% of pts with CML-CP have at least one comorbidity at diagnosis (Hoffmann et al. Leukemia 2015); as such, comorbidities may influence first-line treatment choice for most pts. In this exploratory post hoc analysis of DASISION at 5-years' follow-up, we investigated the effect of comorbidities on response outcomes with dasatinib vs imatinib.
Methods: DASISION was a multinational, open-label, phase 3 trial assessing dasatinib vs imatinib for newly diagnosed CML-CP. Pts were randomized to receive 100 mg dasatinib (n = 259) or 400 mg imatinib (n = 260) once daily. Charlson Comorbidity Index (CCI) was retrospectively calculated for each pt at diagnosis. Efficacy and safety data were retrospectively stratified based on CCI score categories 2-4, 5-6, and ≥ 7 (higher scores indicate a higher comorbidity burden). Rates of major molecular response (MMR) and molecular response with a 4.5-log reduction (MR4.5) were compared between treatment groups using unstratified Cochran-Mantel-Haenszel tests. Time to MMR and MR4.5 were evaluated using Kaplan-Meier methodology with hazard ratios (HRs) based on Cox proportional hazards models and P values from unstratified log-rank tests. Confidence intervals (CI) for median time to response were calculated using the Brookmeyer and Crowley method. P values are descriptive and unadjusted for multiple comparisons.
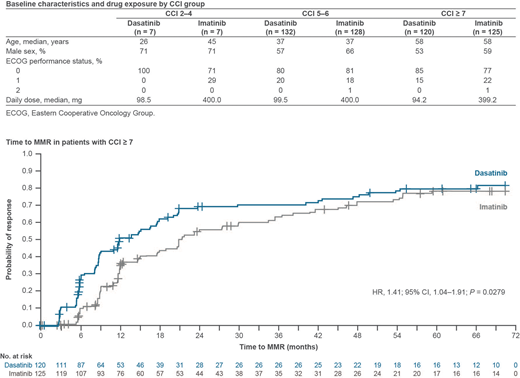
Results: In total, 14 (3%) pts had CCI 2-4 (dasatinib 7, imatinib 7), 260 (50%) had CCI 5-6 (dasatinib 132, imatinib 128), and 245 (47%) had CCI ≥ 7 (dasatinib 120, imatinib 125). Baseline characteristics and drug exposure data are shown in the Table. Dose reductions of dasatinib and imatinib occurred in 14% vs 29% (CCI 2-4 group), 23% vs 12% (CCI 5-6 group) and 54% vs 22% (CCI ≥ 7 group) of pts, respectively. In the CCI 2-4 group, the MMR rate was 85.7% with dasatinib and 57.1% with imatinib (P = 0.2542) and the MR4.5 rates were 71.4% and 14.3% (P = 0.0374), respectively. In the CCI 5-6 group, the MMR rate was significantly higher with dasatinib vs imatinib (81.1% vs 64.8%, P = 0.0033), as was the MR4.5 rate (42.4% vs 29.7%, P = 0.0329). Median time to MMR in the CCI 5-6 group was significantly shorter with dasatinib than imatinib (15.0 vs 24.0 months; HR, 1.64; 95% CI, 1.23-2.19; P = 0.0006). In the CCI ≥ 7 group, response rates were numerically higher with dasatinib than imatinib: MMR rates were 70.8% vs 64.0% (P = 0.2552) and MR4.5 rates were 45.0% vs 39.2% (P = 0.3589), respectively. Median time to MMR was significantly shorter in the CCI ≥ 7 group with dasatinib than imatinib (12.0 vs 21.4 months; HR, 1.41; 95% CI, 1.04-1.91; P = 0.0279; Figure). Grade 3-4 treatment-related adverse events (TRAEs) were reported in 0% vs 14% (dasatinib vs imatinib) of pts with CCI 2-4, 8% vs 9% of pts with CCI 5-6, and 24% vs 13% of pts with CCI ≥ 7. Grade 3-4 TRAEs leading to discontinuation did not occur in the CCI 2-4 group, and were reported with similar frequency with dasatinib and imatinib in both CCI 5-6 (1% vs 1%) and CCI ≥ 7 groups (8% vs 7%). No deaths occurred in the CCI 2-4 group; a similar percentage of pts treated with dasatinib and imatinib died in the CCI 5-6 group (8% vs 9%) and CCI ≥ 7 group (13% vs 11%).
Conclusions: In this exploratory post hoc analysis, dasatinib was effective across all CCI groups, consistent with previous findings suggesting that comorbidities do not negatively affect TKI activity. Molecular response rates were significantly higher with dasatinib than imatinib in pts with CCI 5-6, and comparable between treatments in pts with CCI ≥ 7. Furthermore, time to response was significantly faster with dasatinib than imatinib in both the CCI 5-6 and CCI ≥ 7 groups, while the frequency of grade 3-4 TRAEs leading to discontinuation was similar between treatments. These findings highlight the benefit of first-line treatment with dasatinib in pts with CML-CP and a high comorbidity burden.
Study support: BMS.
Breccia:Bristol-Myers Squibb/Celgene: Consultancy, Honoraria; Abbvie: Consultancy; Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Mauro:Sun Pharma/SPARC: Research Funding; Pfizer: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding. Jabbour:BMS: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding. Saglio:Pfizer: Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; Roche: Research Funding; Ariad: Research Funding; Novartis: Research Funding. le Coutre:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria. DeGutis:Bristol-Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Khan:Bristol-Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Sy:Bristol-Myers Squibb: Current Employment. Swanink:Bristol-Myers Squibb: Current Employment. Cortes:Amphivena Therapeutics: Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Immunogen: Research Funding; Merus: Research Funding; Bristol-Myers Squibb: Research Funding; Arog: Research Funding; BiolineRx: Consultancy, Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Telios: Research Funding; Sun Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal